Bohr Model Drawing Of Oxygen at GetDrawings Free download
Figure 7.3.2 7.3. 2: The emission spectra of sodium and mercury. Sodium and mercury spectra. Many street lights use bulbs that contain sodium or mercury vapor. Due to the very different emission spectra of these elements, they emit light of different colors. The lines in the sodium lamp are broadened by collisions.

Oxygen atom Bohr model stock vector. Illustration of white 267662185
This classical mechanics description of the atom is incomplete, however, since an electron moving in an elliptical orbit would be accelerating (by changing direction) and, according to classical electromagnetism, it should continuously emit electromagnetic radiation.
Oxygen Electronic Structure lupon.gov.ph
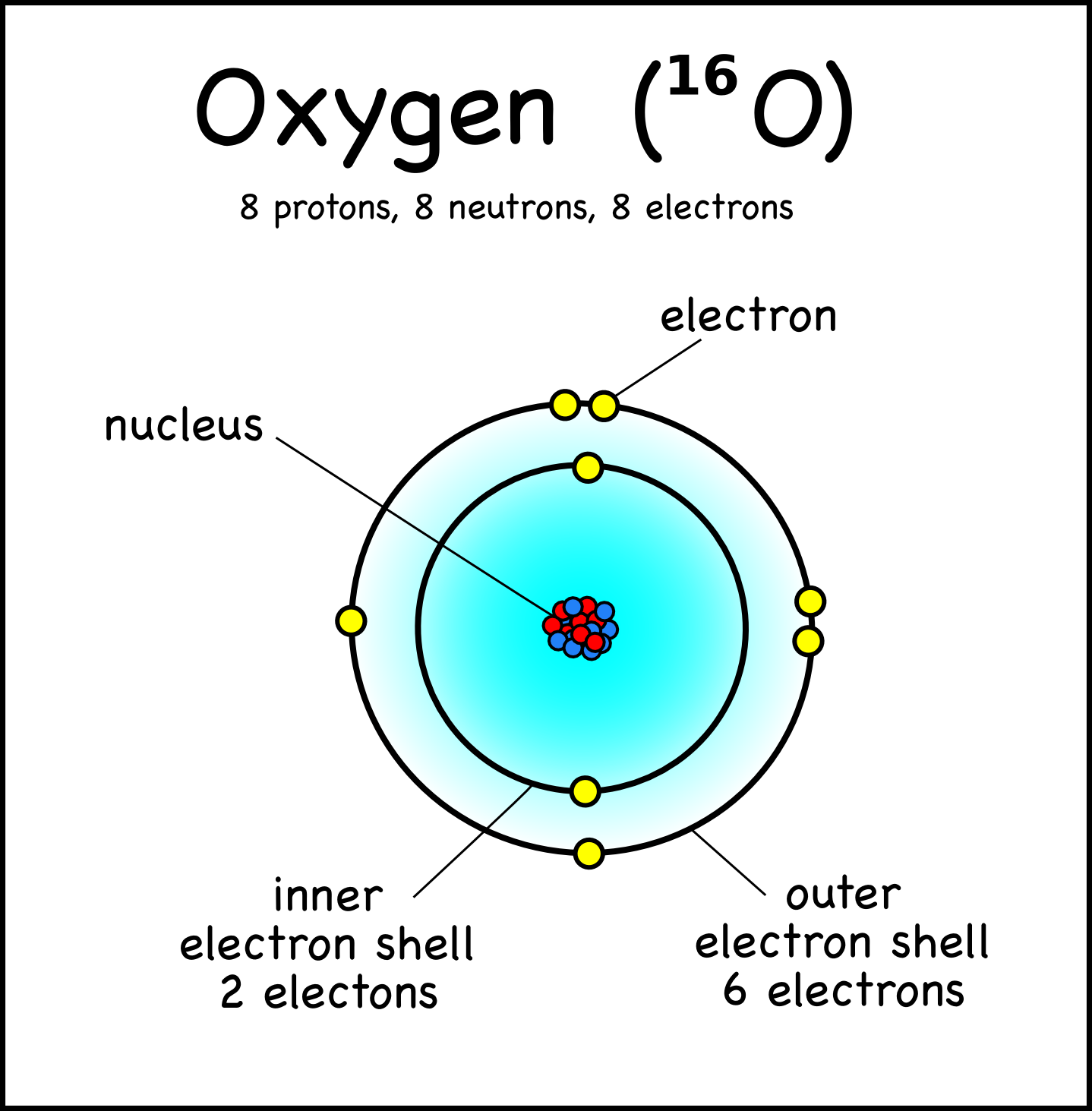
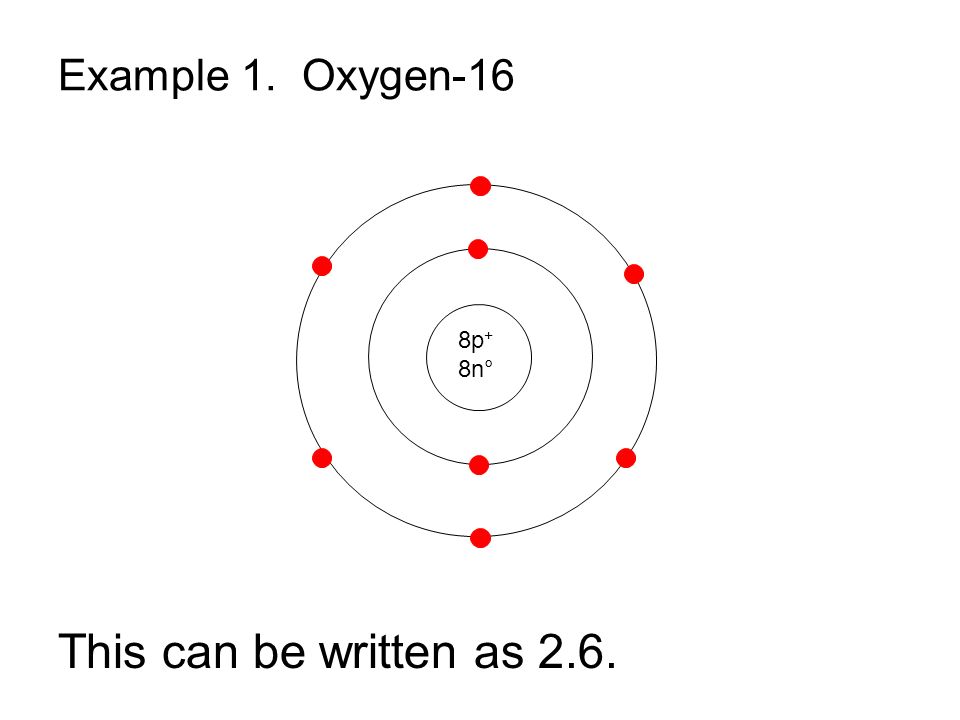
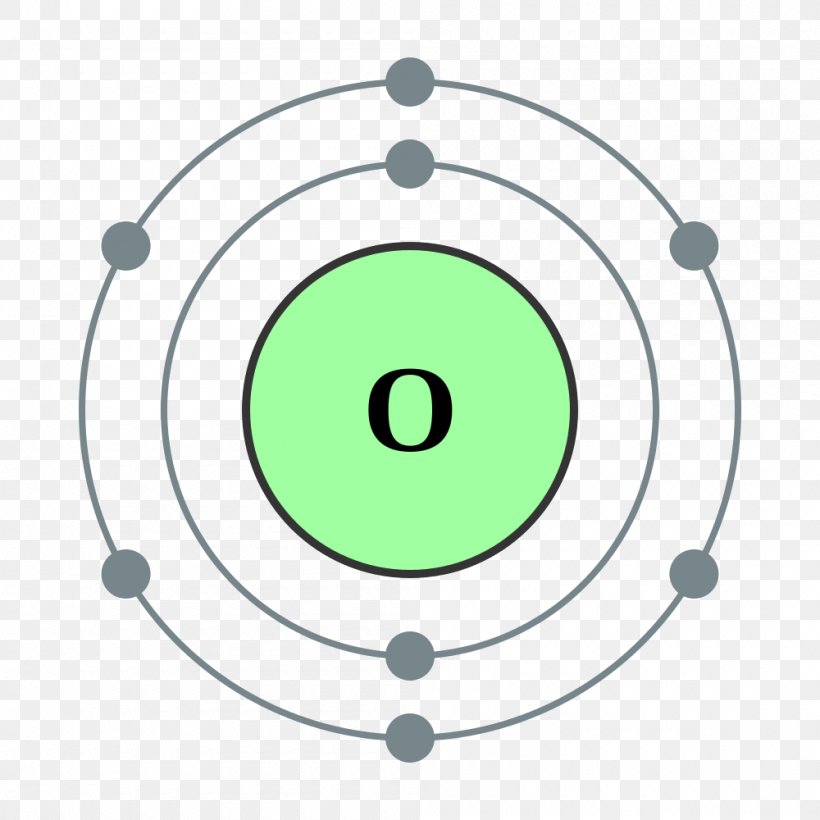
How to draw the Bohr-Rutherford Diagram for Oxygen. 2 electrons can go in the first shell, 8 in the second, 8 in the third, and so on.

Oxygen, atom model. Chemical element with symbol O and with atomic number 8. Bohr model of
One of the weaknesses of Bohr's model was that he could not offer a reason why only certain energy levels or orbits were allowed. Figure 10.4.1 10.4. 1: The energy levels of the electrons can be viewed as rungs on a ladder. Note that the spacing between rungs gets smaller at higher energies (CC BY-NC; Ümit Kaya)
Bohr Model Chemical Element Oxygen Atomic Theory PNG, Clipart, Angle, Area, Atom, Atomic Number
The Bohr Model is known as a planetary model because these orbits look similar to that of planets orbiting the sun. The Bohr Model, Explained There are three main factors that characterize the Bohr model. First, in the Bohr Model, the orbits have a set size and energy.

draw a bohr model of an oxygen atom artvansaletoday
This page titled 5.1: The Bohr Model is shared under a CC BY-SA 4.0 license and was authored, remixed, and/or curated by Tom Weideman directly on the LibreTexts platform. Back in the early days of quantum mechanics, Bohr constructed a model of the hydrogen atom that worked surprisingly well considering its simplicity.

Oxygen Bohr Model (Diagram, Steps To Draw) Techiescientist
Oxygen Bohr Model (Diagram, Steps To Draw) Oxygen is a non-metal and belongs to the Chalcogens i.e. 16 th group of the Periodic table. It has the atomic number 8 and is denoted by the symbol O. It occurs as a diatomic gas and is also the third most abundant element in the universe. It is a colorless and odorless gas with oxidizing properties.
Bohr Model Drawing Of Oxygen at Explore collection of Bohr Model Drawing Of
Answer. Bohr's model of the hydrogen atom provides insight into the behavior of matter at the microscopic level, but it does not account for electron-electron interactions in atoms with more than one electron. It does introduce several important features of all models used to describe the distribution of electrons in an atom.
Oxygen Atom Diagram Photos Cantik
The Bohr model represents these energy levels as rings. We can tell that the two electrons in the model above are at the same energy level because they are on the same ring. Limitations. By representing electrons as particles, the Bohr model does not reflect the wave properties of electrons. The electrons appear to exist in specific locations.

Oxygen atom bohr model Royalty Free Vector Image
Category: Science & Tech Key People: Niels Bohr Related Topics: atom On the Web: Space.com - The Bohr model: The famous but flawed depiction of an atom (Jan. 08, 2024) See all related content → Bohr model, description of the structure of atoms, especially that of hydrogen, proposed (1913) by the Danish physicist Niels Bohr.

Diagram representation of the element oxygen Vector Image
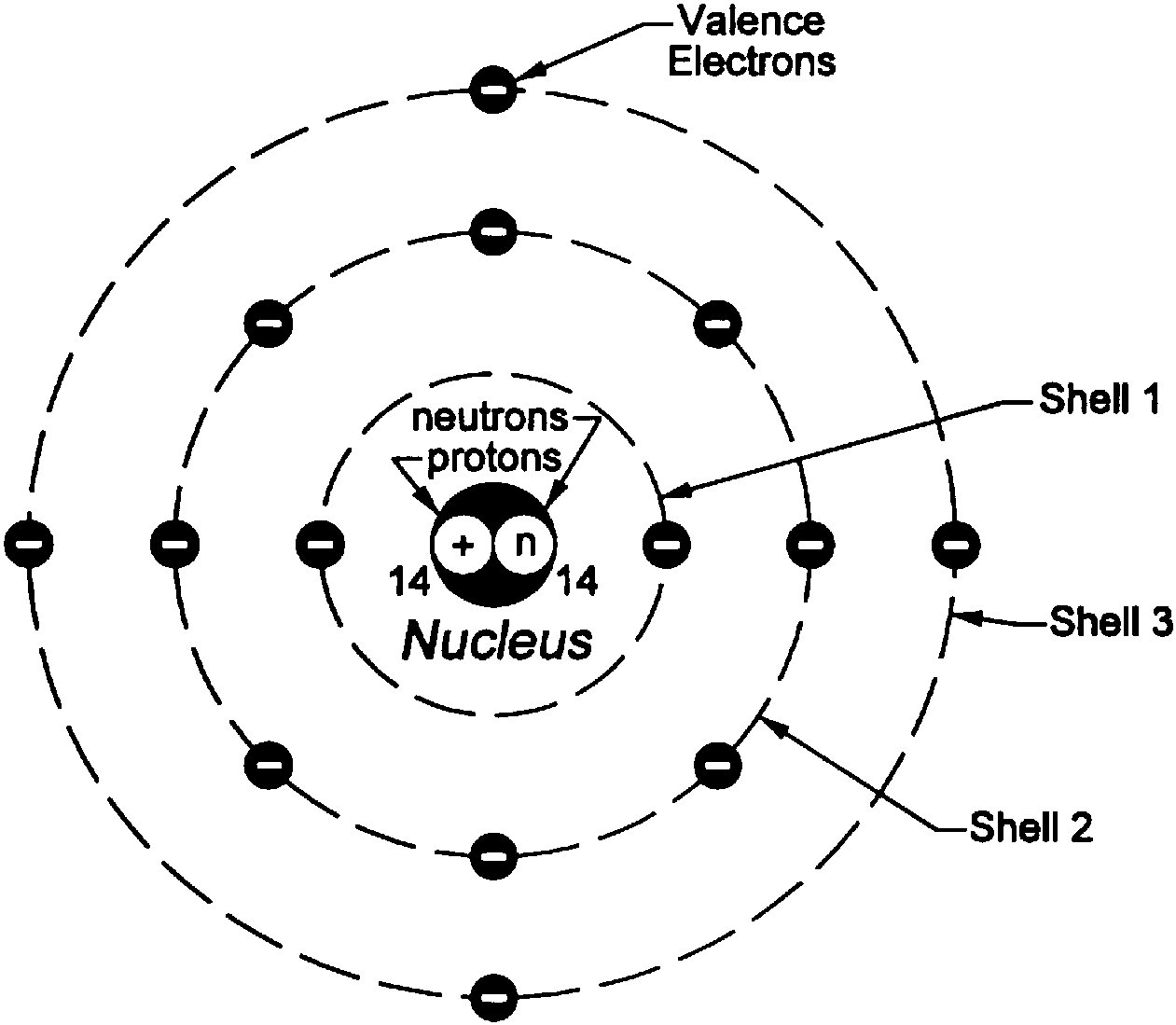
A Model for Atomic Structure: An atom is the smallest particle that makes up matter. It has subatomic particles within it called protons, neutrons, and electrons. The Bohr model, named after scientist Neils Bohr, is a way of illustrating the structure of the atom and the location of its subatomic particles. Specifically, the model shows that.
Oxygen (O) AMERICAN ELEMENTS
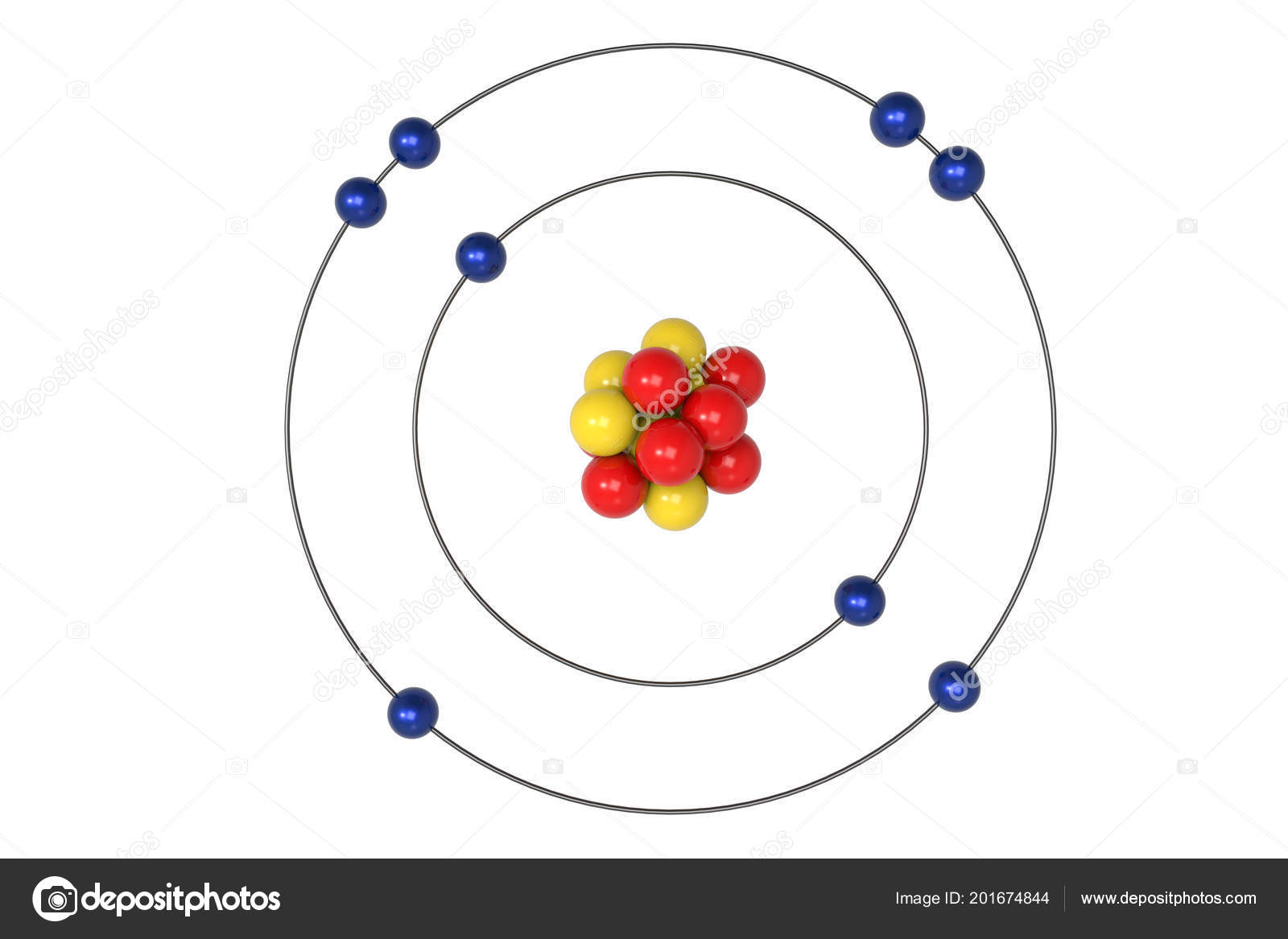
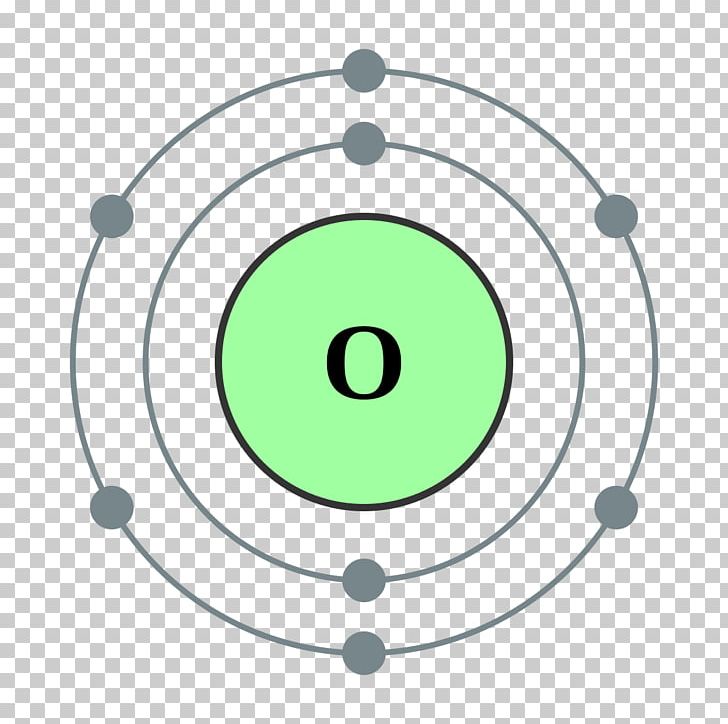
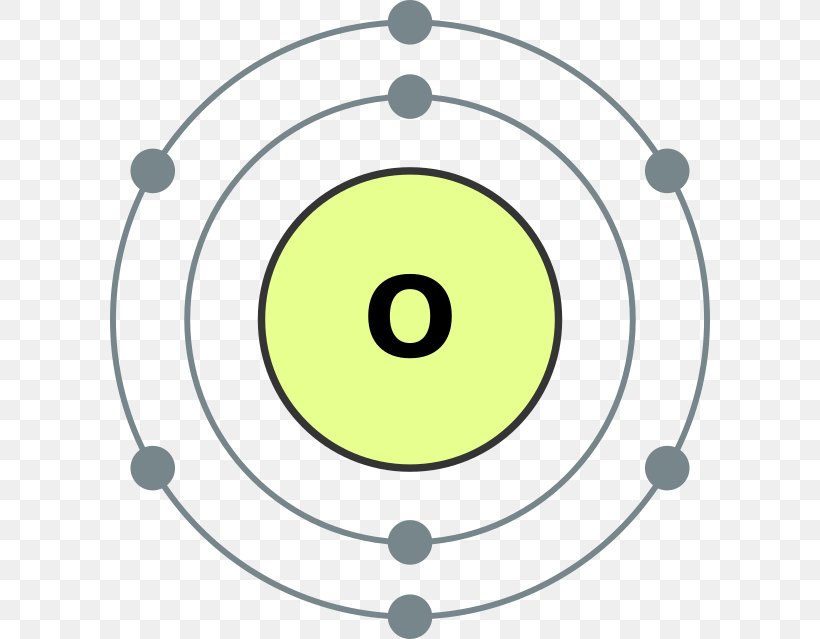
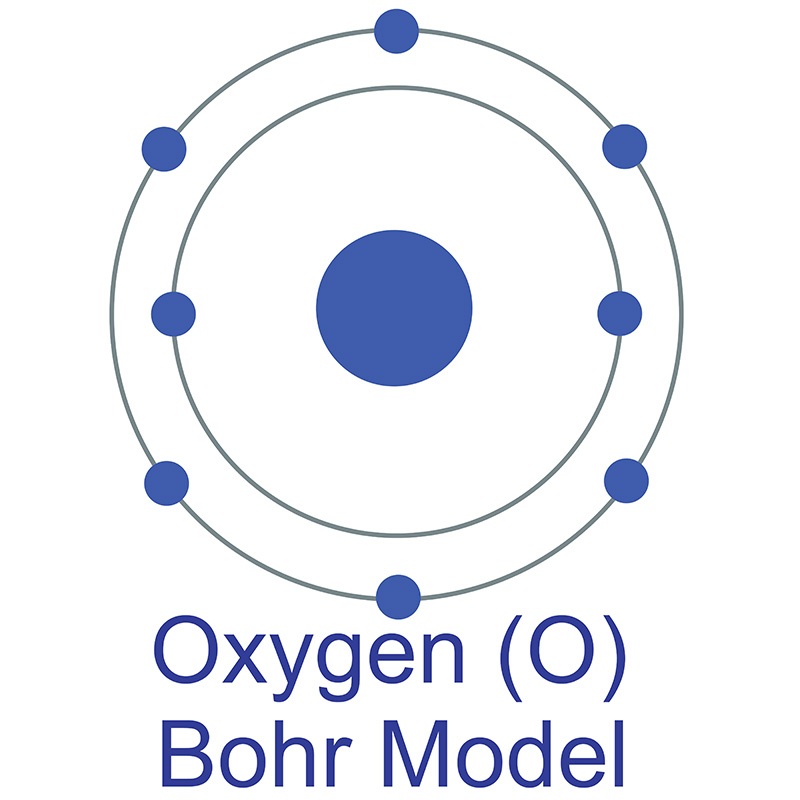
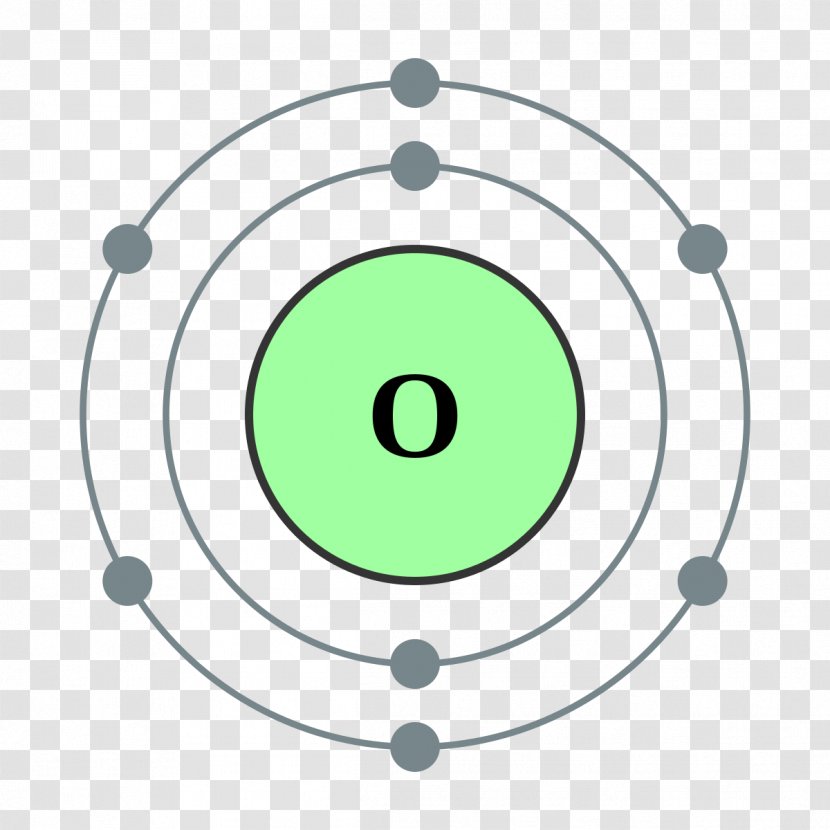
The Bohr model of oxygen contains a nucleus having 8 protons and 8 neutrons in the center, and around this nucleus, there are two electron shells containing 8 electrons. Atomic Structure (Bohr Model) for Oxygen (O) Watch on Contents Steps #1 Write protons, neutrons, and electrons of oxygen atom #2 Draw nucleus of oxygen atom
Bohr Model Drawing Of Oxygen at GetDrawings Free download
Apply: atomic spectra Science > High school chemistry > Atoms, elements, and the periodic table > The Bohr model and atomic spectra Apply: Bohr models Google Classroom A Bohr model of an oxygen atom is shown below. How many valence electrons does the oxygen atom have? Choose 1 answer: A B C Stuck? Review related articles/videos or use a hint.
Bohr Model Chemical Element Oxygen Atomic Theory Green Shells Transparent PNG
Explaining the Bohr Model. In 1924 Louis de Broglie proposed that electrons have a wave nature. As part of that proposal he also described the relationship between the wavelength of the wave aspect and the mass and speed of its particle aspect. The proposal has been experimentally confirmed and is one of the fundamental aspects of Quantum.
Bohr Model Oxygen Chemical Element Atomic Number, PNG, 1000x1000px, Bohr Model, Area, Atom
Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus. Bohr's model calculated the following energies for an electron in the shell, n : E ( n) = − 1 n 2 ⋅ 13.6 eV

Bohr Model Drawing Of Oxygen at GetDrawings Free download
The Bohr model is a relatively primitive model of the hydrogen atom, compared to the valence shell model. As a theory, it can be derived as a first-order approximation of the hydrogen atom using the broader and much more accurate quantum mechanics and thus may be considered to be an obsolete scientific theory.